Learning Outcomes:
i. Explain the relationship between the outermost s and p orbital system of an element and its chemical properties.
ii. Analyze the trends in ionization energy, electronegativity, and metallic character across periods and groups of the periodic table.
iii. Connect the electronic configuration of an element to its ability to form specific types of bonds, such as ionic, covalent, and metallic bonds.
iv. Apply the concept of effective nuclear charge (Zeff) to explain the variations in ionization energy and electronegativity across the periodic table.
Introduction
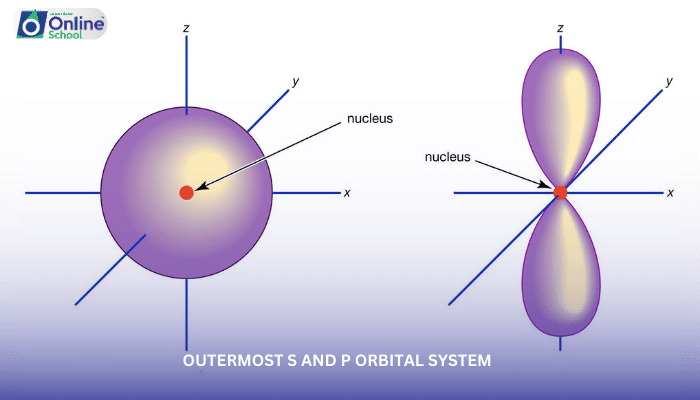
The outermost s and p orbitals of an element play a crucial role in determining its chemical properties. These orbitals dictate the electron arrangement and the ability of an element to form bonds with other atoms. In this lesson, we will delve into the relationship between the outermost s and p orbital system and the chemical properties of elements, exploring the trends across periods and groups of the periodic table.
i. Orbital Occupation and Chemical Properties
The occupation of the outermost s and p orbitals directly influences the chemical behavior of an element. The number of valence electrons, the electrons involved in bonding, is determined by the filling of these orbitals.
Alkali metals: Group 1 elements have one valence electron in the outermost s orbital. This electron is readily lost, resulting in the formation of ionic bonds with electronegative elements. Alkali metals are highly reactive, forming ionic compounds with nonmetals.
Alkaline earth metals: Group 2 elements have two valence electrons in the outermost s orbital. These electrons are also easily removed, leading to the formation of ionic bonds with electronegative elements. Alkaline earth metals are less reactive than alkali metals due to their increased nuclear charge.
Transition metals: Transition metals have varying electron configurations in their d orbitals. The number of valence electrons can range from two to six. Transition metals exhibit diverse bonding behavior, forming ionic, covalent, and metallic bonds depending on the specific element and its oxidation state.
Halogens: Group 17 elements have seven valence electrons, with one electron occupying the outermost p orbital and six electrons in the lower-energy p orbitals. Halogens are highly electronegative and readily gain electrons, forming ionic bonds with electropositive elements.
Noble gases: Group 18 elements have a complete set of electrons in their outermost s and p orbitals, resulting in a stable electron configuration. Noble gases are chemically inert, exhibiting very low reactivity due to their filled valence orbitals.
ii. Trends Across Periods and Groups
The properties of elements exhibit distinct trends across periods and groups of the periodic table. These trends can be explained by the changes in electron configuration and the effective nuclear charge (Zeff), the perceived charge experienced by an electron in an atom.
Ionization energy: Ionization energy generally increases across a period and decreases down a group. This trend is attributed to the increasing Zeff across a period and the decreasing Zeff down a group.
Electronegativity: Electronegativity generally increases across a period and decreases down a group. This trend is also related to the changes in Zeff, with higher Zeff leading to stronger attraction of electrons and higher electronegativity.
Metallic character: Metallic character generally decreases across a period and increases down a group. This trend is due to the increasing number of electrons in the outermost energy level, which become more readily available for bonding with neighboring atoms.
iii. Connection to Bond Formation
The electronic configuration of an element dictates its ability to form specific types of bonds. Ionic bonds, characterized by the transfer of electrons, are favored by elements with large differences in electronegativity. Covalent bonds, involving the sharing of electrons, are formed between elements with similar electronegativity. Metallic bonds, characterized by a delocalized sea of electrons, are prevalent among transition metals.
iv. Effective Nuclear Charge (Zeff)
The concept of effective nuclear charge (Zeff) helps explain the variations in ionization energy and electronegativity across the periodic table. Zeff is the perceived charge experienced by an electron in an atom, considering the shielding effect of other electrons. Increased Zeff results in stronger attraction of electrons, leading to higher ionization energy and electronegativity.
The outermost s and p orbital system of an element plays a fundamental role in determining its chemical properties. Understanding the relationship between electron configuration and chemical behavior provides a deeper insight into the periodic trends and the diverse bonding patterns observed among elements.